This case has gone through all instances in the Norwegian court system, with the Norwegian Supreme Court rendering its first decision on colour marks in December 2017. Overall, the courts find that neither purple, nor specific shades of purple, have protection as unregistered trade marks for inhalers for GlaxoSmithKline in Norway.
In 1999 GlaxoSmithKline (GSK) launched the very successful inhaling medicine Seretide.The colour purple has been used prominently with the product, together with signs such as Seretide, Diskus and GSK. The inhaler itself comes in two shades of purple, Pantone 2587C (“GSK dark purple”) and 2567C (“GSK light purple”).
In 2014, Sandoz launched a generic version of Seretide, Airflusal Forspiro. The inhaler comes in a different shade of purple compared to that of Seretide.
 |
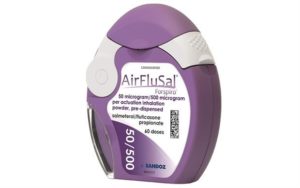 |
| Seretide | Airflusal Forspiro |
GSK filed a suit in 2014, claiming that Airflusal Forspiro inter alia infringes GSK’s rights to their unregistered trade mark rights to the colour purple, alternatively to the unregistered trade marks for GSK dark and light purple.
GSK does not have any trade mark registration for the colour purple in Norway, and therefore had to base their trade mark claims on acquired unregistered trade mark rights.
The main issue before the Appeal Court was whether GSK had acquired trade mark rights to purple, while the main issue before the Supreme Court was whether GSK dark purple had acquired distinctiveness.
In order to acquire rights to an unregistered trade mark in Norway, the mark must be well-known amongst the relevant public. The threshold level for “well-known” is quite high.
Furthermore, obtaining trade mark protection for colours is not easy. Colours are not usually perceived as an indication of commercial origin. However it is possible that a colour mark may “toil itself to distinctiveness” through use, as it is so poetically described in the judgment of the Supreme Court.
To make matters even worse for GSK, they had to disprove claims that the colour purple was used descriptively for combination inhalers.
Based on extensive evidence, the Appeal Court concludes that there exists and has existed an informal colour system for inhalers. The colour blue is used for relievers, the colours red, orange or brown are used for preventers, while purple is used for combination products such as Seretide.
Not only is the colour purple used for combination products, the Appeal Court finds that shades of purple are used to indicate the strength of the combination products. Lighter shades indicate lower concentration, while darker shades indicate a higher dose.
On the basis of this, the Appeal Court finds that the colour purple has not acquired distinctiveness as a source identifier.
Before the Supreme Court, market surveys were of particular importance when determining if Pantone 2587C has become well known.
The Supreme Court corroborates the previous findings of descriptive use, and notes that although it is possible to prove actual knowledge of the mark among the consumers, it might be much more difficult to prove that the relevant consumer perceives this use as an indication of commercial origin.
The relevant public was a matter of dispute before all instances. The Supreme Court defines this as a question as to whom may directly or indirectly influence the decision of purchase. The users of the products have chronic sufferings, and will have a particular interest in the choice of medicines. Therefore, the relevant public for these inhalers not only consists of pharmacists and doctors, but also of the end consumers.
According to the Supreme Court, the market surveys show that knowledge of GSK’s use of the colour purple is low among the end users.
The Supreme Court accepts that the knowledge of Pantone 2587C for Seretide is high amongst pharmacists and doctors. However, they also find that this part of the public does not perceive the colour as a source identifier. The extensive knowledge of the colour purple is put down to its informative and descriptive use for Seretide inhalers manufactured by GSK. Even though the knowledge is high amongst the professional public and the colour may be associated with Seretide, the colour will therefore not be perceived as an indication of commercial origin.
This case serves to illustrate how difficult, almost impossible, it can be to substantiate that a colour is perceived as a source identifier when the colour may have a descriptive function.
GSK has also had its spot of bother with the dark purple before the EUIPO. EUTM 3890126 of the purple inhaler has been cancelled, while EUTM No. 014596951, GSK dark purple, is pending before the Boards of Appeal after having been refused by EUIPO as non-distinctive under Article 7(1)(b) EUTMR.
_____________________________
To make sure you do not miss out on regular updates from the Kluwer Trademark Blog, please subscribe here.



